Silicone hydrogel contact lenses, made with the help of chlorine chemistry, are some of the most popular “soft contacts” on the market.
 Contact Lenses: Comfort and Vision
Contact Lenses: Comfort and Vision
Contact lenses are a convenient, practically invisible vision-correcting option for people who prefer not to wear glasses. Thanks to technological advances over the past sixty years, today’s contact lenses are more comfortable and convenient than ever.
Approximately 40 million Americans wear contact lenses. Reasons for choosing contacts range from preferences of appearance, to the requirements of sports, to the desire for a wider field of view. Most people adapt readily to wearing contacts and enjoy seeing the world clearly without glasses.
The Quest for Lenses that “Breathe”
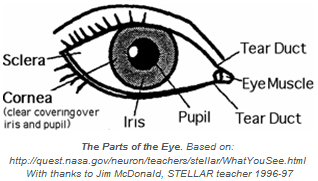
Contact lenses are thin, clear, curved lenses that are inserted over the cornea of the eye to correct vision. The cornea itself is a clear natural window that covers the iris and pupil (see the diagram above).
Contact lens technology has come a long way since 1949 when the first modern contacts were developed. While most contact lenses in use today are made of soft synthetic materials, early “hard contacts” were produced from a stiff plastic called polymethyl methacrylate (PMMA).
For good eye health, it is important that oxygen be allowed to flow to the cornea (see the diagram). Covering the cornea with a rigid, impenetrable material, such as PMMA contact lenses, prevents this flow and raises the risk of dryness. The quest for “breathable lenses” that permit oxygen flow to the cornea has driven much research over the past decades.
A solution to the “oxygen flow” problem came from the work of a Czech chemist in the late 1950’s. Otto Wichterle’s work on “biological gels” led to the development of “hydrogel” contact lenses. Hydrogels are superabsorbant polymers that can absorb large amounts of water without dissolving. Hydrogels have found use in everything from contact lenses to human tissue repair.
The water in hydrogel contacts carries oxygen through the lens to the eye. Hydrogel lenses debuted in the early 1970’s. While they represent a vast improvement over PMMA lenses, hydrogels were found to dehydrate at a rate that increased with the level of water contained in them. This reduces the length of time over which these lenses can be worn on a daily basis.
Silicone + Hydrogel = Breathable Combination
In 1999, the transmission of oxygen or “breathability” of lenses was improved dramatically with the introduction of silicone hydrogel lenses. The silicone polymer consists of a long string of repeating chemical units, known as monomers. Unlike the polymers used in other hydrogels that rely on water to transport oxygen, silicone’s polymer structure includes open “channels” through which oxygen gas can flow. This feature permits between six and seven times more oxygen to pass through silicone hydrogels than through other hydrogels—a significant improvement in contact lens technology.
Silicon vs. Silicone: What a difference an “e” Makes
Silicon, number 14 on the Periodic Table, is one of the naturally-occurring chemical elements, which are the building blocks of matter. Silicone, ending with an “e,” is a compound consisting of repeating units of silicon and oxygen.
Chlorine Chemistry Makes Silicone Possible
Chlorine chemistry is essential to producing silicone, a substance that not only makes “breathable” contact lenses possible, but is also the basis of a wide variety of greases, sealants, adhesives, caulk, specialty rubbers, waterproofing agents, glazes, coatings and insulators.
A 2006 analysis estimated that the annual economic benefit of silicone products to U.S. and Canadian consumers is approximately $1 billion. Silicones are heat-resistant and resistant to chemical attack. New “no-slip” mixing bowls and heat resistant spatulas, baking dishes and oven mitts are silicone products. Wrist bands of silicone are extremely popular. “Silly Putty®” is a silicone-based plastic toy invented by a General Electric engineer. Its bouncing, stretching and printed image-copying properties delight and amaze children. When it appeared in 1949, Silly Putty® sold faster than any other toy in previous history.
Silicone Polymers
On the molecular level, silicone consists of billions of chains of alternating silicon and oxygen atom “links.” Silicones are unique in that many manufactured polymers, such as plastics, are based on carbon and hydrogen. Natural polymers of silicon and oxygen are extremely common on Earth, however, and form the basis of many of Earth’s minerals, including the feldspars of the planet’s crust.
Scientists control the properties of silicone polymers by varying the length of silicon-oxygen chains, installing connections between chains (known as cross-linking) or by adding chemical groups to the chains, as one might add charms to a bracelet. In manipulating these polymers chemically, silicones can be engineered to be anything from liquid to gel to rubber to hard plastic.