Chlorine chemistry helps produce this versatile ingredient found in everyday products, from paints to paper to sunscreen.
 Is your room painted purple or bright orange? If you have ever had the pleasure of picking your own wall paint or paint for any project, you know that today’s paints are available in an amazing array of hues. For that you can thank the color-imparting chemistry of pigments. And a very important pigment used in paints today—titanium dioxide—is produced with the help of chlorine chemistry.
Is your room painted purple or bright orange? If you have ever had the pleasure of picking your own wall paint or paint for any project, you know that today’s paints are available in an amazing array of hues. For that you can thank the color-imparting chemistry of pigments. And a very important pigment used in paints today—titanium dioxide—is produced with the help of chlorine chemistry.
Versatile Compound
Titanium dioxide is the most common white pigment used in paints, coatings, plastics, paper, inks, fibers, foods and cosmetics. Stable, nonflammable and non-toxic, this compound has replaced compounds of lead, a toxic element, as the most common white pigment used in residential paints. Titanium dioxide is also used to provide UV protection in sunscreens and cosmetics. Chlorine chemistry is used to transform and purify processed titanium ore into titanium dioxide using a method that largely recycles chlorine. A recent economic analysis determined that using chlorine chemistry to produce titanium dioxide saves U.S. and Canadian consumers at least $1.4 billion per year compared to alternate technologies.
Naturally Abundant
The chemical element titanium is the ninth most abundant element in the Earth’s crust. Titanium is not found chemically uncombined in nature. It is commonly bonded to oxygen in the form of titanium dioxide.
Natural titanium dioxide is very impure, however, and is found mixed with oxides of iron, magnesium and manganese, among other elements. Titanium sands, one of the main sources of titanium dioxide, in fact, are black due the presence of abundant impurities, typically iron.
Titanium Dioxide—Extraordinary Optics
In addition to its natural abundance and lack of toxicity, titanium dioxide is a popular pigment because of its optical properties—the way it interacts with light. Titanium, atomic number 22 on the Periodic Table of the Elements, contains 22 protons and electrons, making it “electron rich.” When a beam of light falls on a solid particle of titanium dioxide, the light is dramatically slowed down by the interference of titanium’s many electrons and by titanium dioxide’s unique internal arrangement of atoms (see diagram).
Light really “puts on the brakes” when it encounters the atomic grid--or crystal structure--of titanium dioxide. Imagine shining a flashlight into a dark room full of mirrors. As light reflects from one mirror to the next, the entire room brightens. This is the property of titanium dioxide that makes it a wonderful pigment. It “traps” and reflects light better than almost any substance known to man. Additions of only small amounts of colorful pigments are required to develop vibrant color in this highly reflective substance.
Grades of titanium dioxide, based on degrees of brightness and whiteness, are made by adjusting its particle size, a factor that has a major effect on the degree of light scattering.
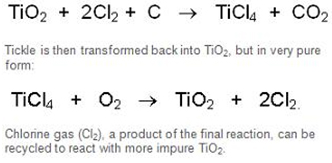
From Black Sands to White Pigment
Chlorine gas is reacted with impure TiO2 and carbon at high temperature to form “tickle” (titanium tetrachloride,TiCl4) according to the reaction: