Chlorine is one of the most abundant chemical elements on Earth. It is ubiquitous in soils, minerals, plants and animals. Seawater is a huge reservoir of dissolved chlorine weathered from the continents and transported to the oceans by Earth's rivers.
Chlorine is also one of the most useful chemical elements. Each chemical element has its own set of unique properties and chlorine is known as a very reactive element—so reactive, in fact, that it is usually found combined with other elements in the form of compounds. More than 3,500 naturally occurring chlorinated organic (associated with living organisms) compounds alone have been identified.
Chlorine's chemical properties have been harnessed innovatively for good use. For example, this element plays an essential role in public health. Chlorine-based disinfectants are capable of removing a wide variety of disease-causing germs from drinking water and wastewater as well as from hospital and food production surfaces. Additionally, chlorine plays a critical role in the manufacture of thousands of products we depend upon every day, from computer chips to crop-protection chemicals to cancer-fighting drugs. Some of these products contain chlorine, and others simply depend on chlorine chemistry for an intermediate step in their manufacture. As the ninth largest chemical produced in the U.S. by volume, chlorine is truly a "workhorse chemical."
Released From the Salt of the Earth…
Chlorine is produced industrially from the compound sodium chloride, one of the many salts found in geologic deposits formed from the slow evaporation of ancient seawater. When electricity is applied to a brine solution of sodium chloride, chlorine gas (Cl2), caustic soda (NaOH) and hydrogen gas (H2) are generated according to the following reaction:
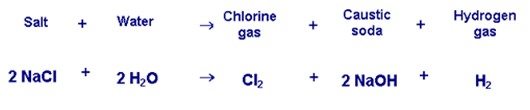
As the reaction demonstrates, chlorine gas cannot be produced without producing caustic soda, so chlorine and caustic soda are known as "co-products," and their economics are inextricably linked. Caustic soda, also called "alkali," is used to produce a wide range of organic and inorganic chemicals and soaps. In addition, the pulp and paper, alumina and textiles industries use caustic soda in their manufacturing processes. Thus, the "chlor-alkali" industry obtains two very useful chemicals by applying electrical energy to sea salt.
Conclusions
- Chlorine is one of nature's most common chemical elements.
- Electricity applied to salt solutions enables the chlor-alkali industry to harness chlorine captured in salt deposits of ancient oceans.
- Chlorine's chemical properties make it an extremely effective disinfectant and essential component in the chemical manufacture of literally thousands of vital products used every day.
Related Materials