Chlorine chemistry helps relieve and control asthma symptoms for over 20 million Americans.
 Common activities can be frustrating for people with asthma. A chronic inflammation of the air passages of the lungs, asthma can force adults and children onto the sidelines of normal activity. According to the U.S. Centers for Disease Control and Prevention, over 16 million adults and nearly 7 million children (about 7.5 percent of the U.S. population) have asthma. While there is no cure for asthma, its symptoms can be managed, guided by resources such as the American Lung Association Breathe Well, Live Well program and the Nemours Foundation’s Kids Health website.
Common activities can be frustrating for people with asthma. A chronic inflammation of the air passages of the lungs, asthma can force adults and children onto the sidelines of normal activity. According to the U.S. Centers for Disease Control and Prevention, over 16 million adults and nearly 7 million children (about 7.5 percent of the U.S. population) have asthma. While there is no cure for asthma, its symptoms can be managed, guided by resources such as the American Lung Association Breathe Well, Live Well program and the Nemours Foundation’s Kids Health website.
Modern medicinal chemistry provides many of the essential tools prescribed by doctors to control patients’ asthma symptoms. Among these are the pharmaceuticals that reduce inflammation of the air passages and relax tense muscles surrounding the airways. Chlorine chemistry, in particular, plays a role in helping to provide a popular, asthma prevention medication. It also helps produce the propellants that guide inhaled medicines into the lungs to relieve sudden symptoms.
Asthma: Breathing Made Difficult
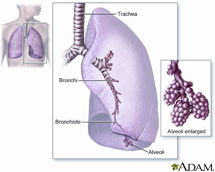 The oxygen we breathe in is used by our bodies to extract energy from food. Exhaling releases carbon dioxide, a waste product of the body’s energy-yielding processes. This important gas exchange occurs deep inside the lungs.
The oxygen we breathe in is used by our bodies to extract energy from food. Exhaling releases carbon dioxide, a waste product of the body’s energy-yielding processes. This important gas exchange occurs deep inside the lungs.
Inhaled air is conditioned for its passage into the lungs by being warmed, filtered and humidified in the intricate channels of the nose. It then enters the main stem of the lung’s airways, known as the trachea. Air continues through small branching airways called bronchi, which give way to even smaller passages called bronchioles. Bronchioles end in grape-like clusters of air sacs known as alveoli. These are the sites of oxygen and carbon dioxide exchange. Blood is enriched with oxygen in the alveoli, enabling its distribution to cells throughout the body. At the same time, carbon dioxide, a waste product of living, is released for exhaling.
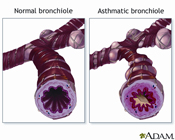 When exposed to certain “triggers,” asthmatics produce a substance called leukotrienes, which cause airways to narrow, swell and fill with mucous, making breathing a struggle. Triggers vary with individuals, but may include pollen, dust, mold, perfume, pets, pollution, cold or hot weather, exercise, or common colds and flu. While there is no fail-safe test for this disease, doctors diagnose asthma when patients experience recurring episodes of wheezing, breathlessness, chest tightness and coughing, particularly at night or in the early morning. An asthma diagnosis is further supported when symptoms are reversible, either spontaneously or with treatment.
When exposed to certain “triggers,” asthmatics produce a substance called leukotrienes, which cause airways to narrow, swell and fill with mucous, making breathing a struggle. Triggers vary with individuals, but may include pollen, dust, mold, perfume, pets, pollution, cold or hot weather, exercise, or common colds and flu. While there is no fail-safe test for this disease, doctors diagnose asthma when patients experience recurring episodes of wheezing, breathlessness, chest tightness and coughing, particularly at night or in the early morning. An asthma diagnosis is further supported when symptoms are reversible, either spontaneously or with treatment.
Asthma medications are of two broad categories: relievers and preventers. Most asthmatics are prescribed a regimen that includes both. Relievers are meant to be used as needed at the first sign of asthma symptoms for preventing “asthma attacks” (before exercise, for example). Preventers are used regularly, whether or not symptoms are evident.
Chlorine Chemistry in Metered Dose Inhalers
Relievers open airways in the lungs with “bronchodilator” medications, delivered directly to the lungs. This is done using a small, portable device known as a metered dose inhaler (MDI), or “puffer.” Hydrofluorocarbon gas propellant (known as either HFC or HFA), a product of chlorine chemistry, ushers a prescribed dose of bronchodilator into the lungs with each puff on the MDI. MDIs are highly effective because they introduce medication precisely and directly into the lungs, where absorption is rapid due to the large surface area of the lungs.
Bronchodilators quickly relax the muscles surrounding the airways, allowing air to flow, reducing noisy symptoms like wheezing and coughing. Nearly 500 million MDIs are manufactured worldwide each year, and they have come to be regarded as the preferred form of delivery of asthma reliever medication. A 2007 study estimated that MDIs save U.S. and Canadian consumers at least $1.2 billion per year, compared to alternative medication delivery systems.
Heroes of Chemistry Develop Asthma Preventer Drug
While relievers work on the spot to relax muscles surrounding airways, they do not prevent asthma attacks. Preventers, including the chlorine-containing medication montelukast sodium, work by blocking the action of leukotrienes.
In 2003, the American Chemical Society (ACS) honored the three Merck research chemists who invented montelukast sodium by naming them “Heroes of Chemistry.” Drs. Robert Young, Robert J. Zamboni and Marc Labelle were recognized by ACS as having helped advance the health, well-being and lifestyles of children around the world. Montelukast sodium is appropriate for children as young as 12 months. In 2003 the Food and Drug Administration approved its use for the relief of seasonal allergies in children as young as two years.
Take Control of Asthma
The National Heart Lung and Blood Institute recommends people with asthma partner with their doctor to develop an Asthma Action Plan. According to the Institute, asthma can be controlled by:
- Taking asthma medicines as prescribed
- Learning what triggers asthma symptoms and taking steps to avoid those triggers
- Tracking the level of asthma control and responding quickly to worsening symptoms
Thanks to the contributions of many heroes of chemistry and medical science, most people with asthma today can lead normal, active lives.
Cracking the Chemistry Naming Code
1,1,1,2,3,3,3-heptafluoropropane is the name of one of the propellants used in metered dose inhalers. It is a hydrofluorocarbon, also called an HFC or an HFA, and chlorine chemistry is used in its production. Have you ever wondered why some chemical names are so long and may even contain strings of numbers? This exercise will help you understand how to crack the chemistry naming code.
Here’s the deal: we start by examining the end of the name.
1,1,1,2,3,3,3-heptafluoropropane
- Notice the word “propane”. Propane is a “chain molecule” in which three carbon atoms are strung together like the links in a chain. The atoms are chemically bonded to one another. Each carbon atom is also bonded to hydrogen. (That makes propane a hydrocarbon, as in fuel, as for the backyard grill). Carbon has four points of attachment, and hydrogen has one, so propane looks like this:
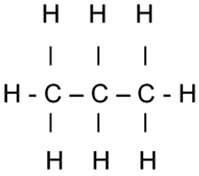
Propane
1,1,1,2,3,3,3-heptafluoropropane
- Having gotten the propane part down, we move slowly toward the left within the long name, and we see “fluoro”. That’s code for the element fluorine, a close neighbor of chlorine on the Periodic Table of the Elements. Now we know there is some fluorine in this compound, but how much, and where are the fluorine atoms positioned along the propane chain?
1,1,1,2,3,3,3-heptafluoropropane
- Creeping along a bit more to the left we can make out “hepta,” Greek for seven. OK, we now know there are seven fluorine atoms somewhere along the chain. It is helpful to know that fluorine, like hydrogen, has one point of attachment.
1,1,1,2,3,3,3-heptafluoropropane
- The time has come to confront the numbers. No, it’s not a combination for a chemistry lab locker, but a kind of address system. Notice that there are seven numbers, one for each fluorine atom. Each number corresponds to a fluorine atom bonded to either carbon 1, 2 or 3 in the propane “chain”. Fluorine atoms replace hydrogen atoms according to the number addresses. “1,1,1” refers to three fluorine atoms on the first carbon atom, and so on.
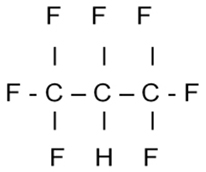
1,1,1,2,3,3,3-heptafluoropropane