Chlorine chemistry helps link the molecules of the tough fibers of protective equipment needed by police, military and emergency responders.
 The brave men and women of the military, law enforcement and emergency response communities deserve the best protective equipment available. Aramid fibers, a class of strong, heat-resistant synthetic fibers produced with the help of chlorine chemistry, go a long way to “protecting the good guys.”
The brave men and women of the military, law enforcement and emergency response communities deserve the best protective equipment available. Aramid fibers, a class of strong, heat-resistant synthetic fibers produced with the help of chlorine chemistry, go a long way to “protecting the good guys.”
Since 1975, Kevlar® and other aramid fiber-containing armor, including bullet-resistant vests, flak jackets and helmets, have helped save the lives of at least 3,000 law enforcement officers.
Following Nature’s Example
For centuries, plant and animal fibers have supplied the raw material for clothing, shelter and other objects of daily living. Examples of our intelligent use of natural fibers include warm blankets of sheep’s wool, fine clothing woven from the silkworm’s threads, houses of wood, baskets of dried grasses, and even the horse hair of a violin bow.
Starting in the 1930s and 40s, scientists began developing synthetic fibers with highly desirable properties. In 1935, DuPont scientist Dr. Wallace Carothers invented nylon, an affordable substitute for costly silk. Nylon was made into parachutes, ropes, tents, tires, ponchos and other necessities for the military in World War II. Other synthetic fabrics followed, including polyester, spandex and, in the 1960s, the very strong aramid fibers—the stuff of bullet-resistant vests and other forms of body armor.
An Amazing New Fiber
In the early 1960s, DuPont research chemist Stephanie L. Kwolek was trying to find the chemical path to a new high-performance fiber. Like Carothers before her, Kwolek worked with substances called polymers, large molecules that at the molecular level look like great bunches of chains or gobs of spaghetti. Like the multicolored beads of a necklace strung together in a repeating pattern, polymers are made by chemically linking together many small units. These units are known as monomers. The particular monomers chosen and the way they are linked together are factors that determine the physical properties of each polymer.
Kwolek experimented with aramids, a class of polymers made of strands of six-sided rings of atoms. She found that under certain conditions, molecules of aramids lined up neatly in a liquid solution like uncooked spaghetti in a long box. To form fibers, polymer liquids are forced through the small holes of a showerlike device called a spinneret. Kwolek’s polymer molecules improved their alignment as they exited the spinneret, and as a result the fibers that came out were remarkably strong.
In 1973, DuPont introduced Kevlar®, a very strong, yet lightweight fabric made of woven aramid fibers, which today is used not only for body armor but also for bicycle tires, racing sails, suspension bridge cables and much more.
The Role of Chlorine in Aramid Fiber Production
In the production of aramid fibers, two different monomers are linked together in an alternating pattern like two different kinds of beads on a string. Chlorine atoms (marked by “Cl” in Figure 1) mark “latch points” on either side of the monomer known as TDC.
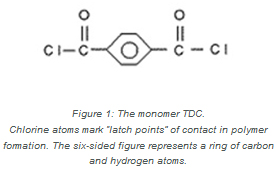
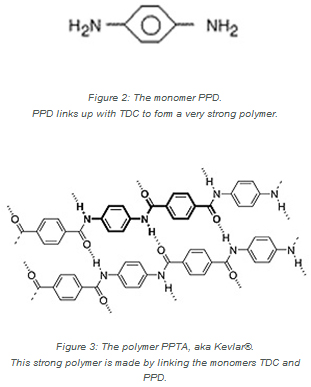
The TDC monomer links up and alternates with another monomer, known as PPD, to make the polymer that DuPont named Kevlar® (see Figure 2). Kevlar® is particularly strong along the length of the fibers due to tight bonding between the monomers.
When TDC and PPD bond together, chlorine from the points of attachment of the monomers are released. The result is that hydrochloric acid (HCl) is a byproduct of the chemical reaction. While chlorine is not found in Kevlar®, it is essential to the production of this life-saving fiber.
A Contribution to Safety
There is no better way to say “thank you” to the good guys who protect us than by providing them with well-designed protective gear.
For her part in developing Kevlar®, Stephanie Kwolek has said she was “elated to have made this discovery because most people work a lifetime, and they never do anything like this—something that saves human lives.” In recognition of her great contribution, Kwolek has received many awards, including the National Medal of Technology Award in 1996, the highest honor for technological innovation in the U.S.